建議您使用以下瀏覽器觀看本網站,
以獲得最佳瀏覽效果。
 TO-O-1002
TO-O-1002
Neovascular/Wet AMD
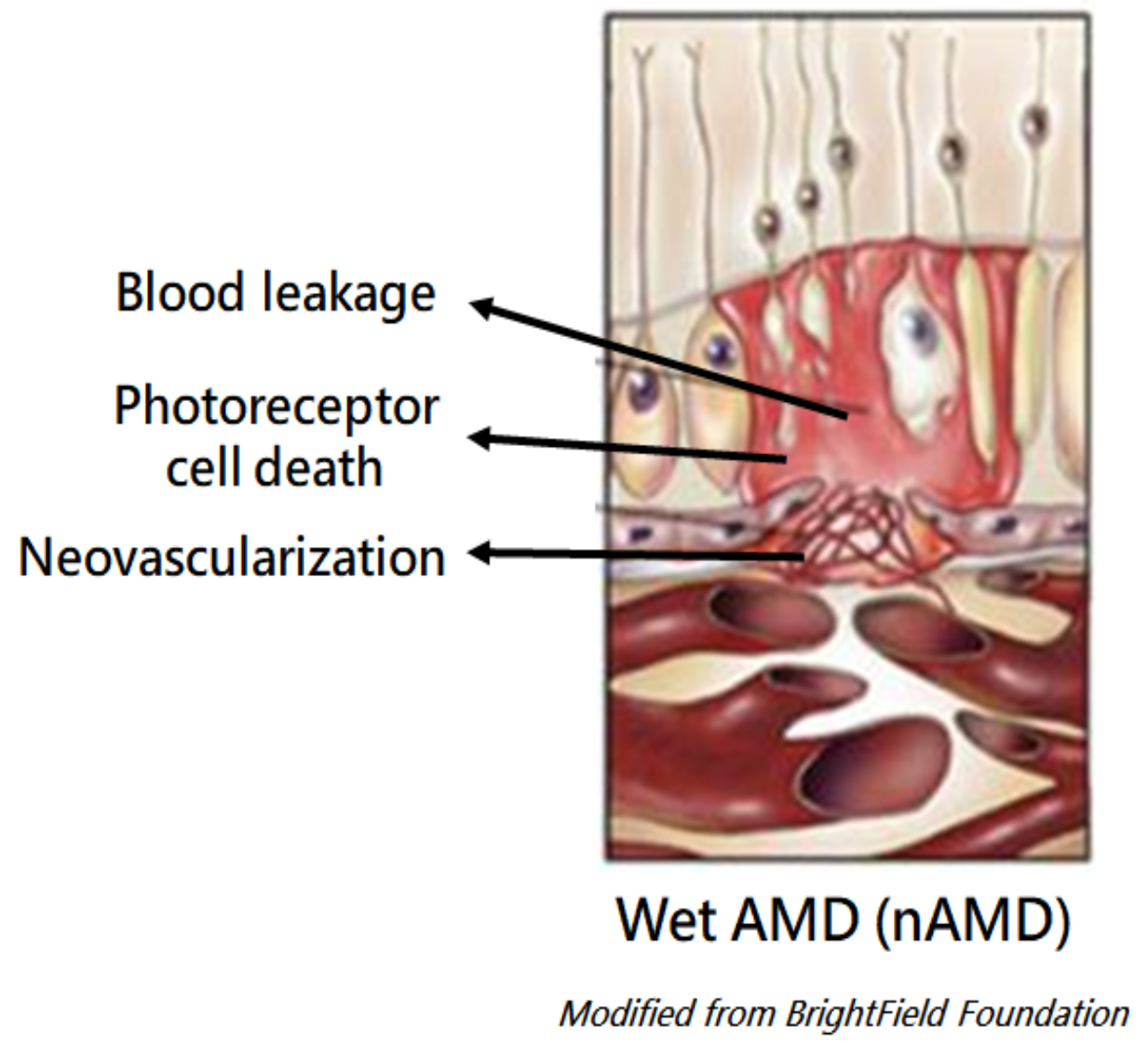
Wet AMD, prevailed in population over the age of 60, accounts for 15% of all AMD. cases. The disease progresses fast due to the leaky and abnormal outgrowth of choroidal vessels beneath the retina. Permanent vision loss could be resulted if proper medical intervention is not introduced. According to the WHO, there are 30 millions of wet AMD patients worldwide in 2022, and will be approaching 36 millions in 2030 a.
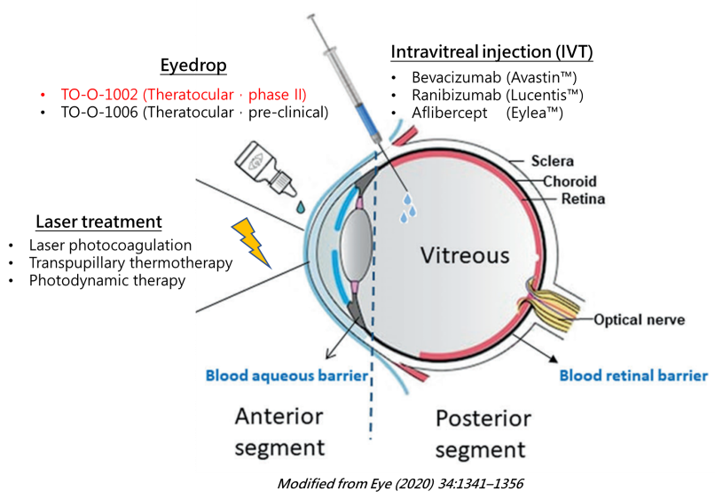
Standard treatments for wet AMD include Laser surgeries and Intravitreal (IVT) injections of anti-VEGF antibodies. IVT injections, although a common procedure, are often costly and very much a office-based procedure performed only by a physician. Patients will be stressed by the needles and the repeated visit schedule for injections. AEs following IVT like injection site pain, bleeding, inflammation or infection are constantly reported. In some severe cases of adverse events, retinal detachment, uprising IOP, or cataract formation are also possible.
Apparently, a novel way of topical ocular delivery of anti-VEGF treatment is not only technically feasible, but also fits patients’ expectation well.
Ref:a. World report on vision. Geneva: WHO; 2019.
nAMD
TO-O-1002 (Formerly known as MG-O-1002) eye drops is a 505b2 type of product. Its active ingredient has been clinically-confirmed with clear anti-VEGF effect, and with no reported corneal toxicity in animal studies. The unique drug product formulation is special designed and tested to deliver enough drug to the targeted lesion sites in the posterior segments of the eyes.
- Repurposing drug, the API has been approved in mainstream countries for years and proved to be safe and effective for human use.
- Will soon be the First Eye Drops formulation ever be brought into wet AMD market for patients’ ease of use and overall therapeutic benefit.
- Patented formulation, great for monotherapy or for at home maintenance phase of current standard IVT treatment.
TO-O-1002 eye drops is targeting 2022/Q3 for its Phase I/II study in patients.









