建議您使用以下瀏覽器觀看本網站,
以獲得最佳瀏覽效果。
 TO-O-1007
TO-O-1007
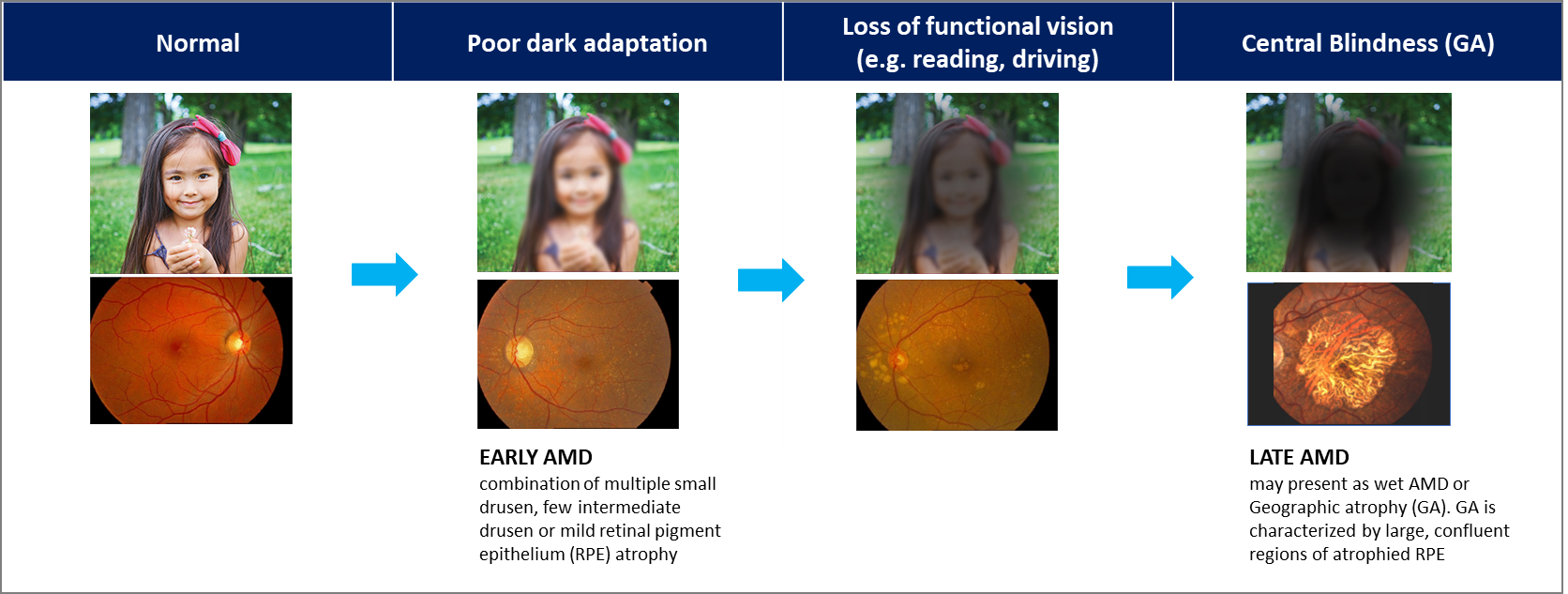
Geographic Atrophy, GA
Dry age-related macular degeneration (dry AMD) is a highly prevalent, age-associated degenerative disease of the macula—which is essential for fine visual acuity and color vision—whose progressive course leads to irreversible and worsening central vision loss, severely impairing daily activities such as reading, recognition, and driving; the global patient population is projected to exceed 300 million by 2040, including more than 1.5 million patients with late-stage dry AMD (geographic atrophy, GA) in the United States and over 5 million worldwide, with prevalence increasing approximately fourfold with each decade after age 50, reflecting a substantial unmet medical need and supporting an estimated global dry AMD market size of USD 10–12 billion annually.
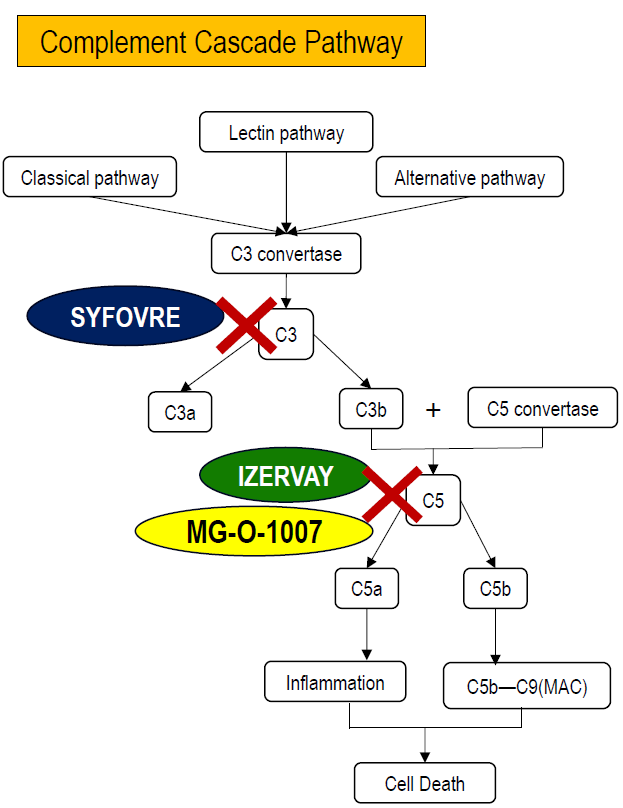
Core Pathogenic Mechanism of Geographic Atrophy (GA)
The pathogenic mechanism of Geographic Atrophy (GA) is closely associated with dysregulated overactivation of the complement cascade. The complement system is a key component of the innate immune response and can be activated through three pathways—the classical pathway, lectin pathway, and alternative pathway—all of which converge on the formation of C3 convertase, leading to the cleavage of C3 into C3a and C3b.
In patients with GA, complement activity remains chronically imbalanced, resulting in sustained activation of C3 and downstream C5. Activation of C3 not only amplifies the overall complement response, but C3b also contributes to the formation of C5 convertase, which cleaves C5 into C5a and C5b. C5a is a potent pro-inflammatory mediator that recruits immune cells and promotes chronic inflammation, while C5b initiates assembly of the membrane attack complex (C5b–C9, MAC), which directly disrupts cell membranes.
Within the retina, prolonged complement activation induces chronic inflammation and progressive cell death of retinal pigment epithelial (RPE) cells and photoreceptors. This process ultimately leads to structural degeneration of the retina, formation of geographic atrophic lesions, and irreversible vision loss.
Accordingly, current therapeutic strategies for GA focus on inhibiting key nodes within the complement cascade. As illustrated, SYFOVRE, a C3 inhibitor, blocks complement activation at an upstream level, whereas IZERVAY and TO-O-1007 target C5-related pathways. By reducing inflammatory signaling and MAC formation, these approaches aim to slow retinal cell loss and delay the progression of GA.
Biodegradable PLGA Implants
PLGA (poly(lactic-co-glycolic acid))–based ocular implants are biodegradable drug-delivery systems developed for treating posterior-segment eye diseases such as retinal and macular disorders. PLGA is a well-established medical polymer that gradually degrades into lactic acid and glycolic acid, which are naturally metabolized by the body, eliminating the need for implant removal and providing a strong safety profile.
These implants are extremely small and are inserted into the vitreous humor using a specially designed injector. Once implanted, they release medication in a controlled and sustained manner over weeks to months. Compared with conventional intravitreal injections, PLGA implants reduce dosing frequency, improve treatment convenience, and lower the risk of injection-related discomfort and infection.
A major advantage of PLGA-based ocular implants is their ability to deliver drugs directly to the target tissues within the eye, enhancing therapeutic effectiveness while minimizing systemic exposure and side effects. By adjusting polymer composition and implant design, the release rate and treatment duration can be tailored to different disease needs.
Overall, PLGA-based ocular implants represent a proven and versatile platform for long-acting ophthalmic therapies, combining biodegradability, sustained drug release, and extensive clinical experience to improve patient outcomes and quality of life.

Geographic Atrophy (GA) – TO-O-1007 Intravitreal Implant
TO-O-1007 intravitreal implant is a 505(b)(2) drug-repurposing, long-acting ocular formulation currently under international patent protection. Its mechanism of action (MOA) is based on inhibition of the C5, thereby aiming to slow the progression of geographic atrophy (GA) associated with dry age-related macular degeneration.
- Clinically validated small-molecule with high tissue penetration and proven safety
- PEG-free, avoiding PEG-related safety risks
- Validated MOA
- Long-acting sustained release with reduced dosing frequency
- Custom injector required (patent pending)
- Ultra-thin implant (~0.3 mm diameter)
- Highly complex manufacturing requiring precise processing and uniform drug distribution
- Limited global GMP manufacturing capability
- High manufacturing and quality-control barriers
- PLGA implants difficult to replicate, creating a high generic barrier
TO-O-1007 is currently under application for clinical trial authorization in Australia, with first-in-human Phase I/IIa studies planned for 2026.










